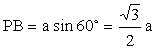The optimal bond angle of Methane |
|||||
|
Introduction Methane
molecule consists of one carbon and four hydrogen atoms (CH4).
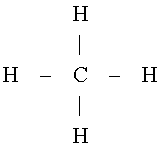
Lewis gave a planar structure for CH4 as shown on the left. This Lewis
structure suggests that the optimal bond angle for methane is 90°. However, this two
dimensional model is unsatisfactory. |
|
||||
|
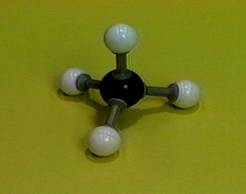
Three dimensional model
The
figure in the left hand side shows a three dimensional model of methane. The central
black ball represents the carbon (C) and four white balls are hydrogen atoms
(H), forming a tetrahedron structure. In this article, we are going to show
that the HCH covalent bond angles are 109.5°. |
|
||||
|
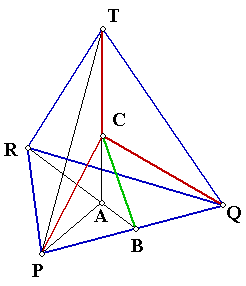
Let
the carbon atom situated at C and four hydrogen atoms at P, Q, R, S. Let
TA be the height of the tetrahedron. The optimal bond angle = Ðx \ Ðx = ÐTCP = ÐTCQ = ÐPCQ B is the mid point of
PQ. ÐRBP = 90° Since PQR is equilateral, ÐPRA = ÐARP = 30° \ ÐPAB = 60° Let PA = a, we can
get |
|
||||
|
Putting PC = y, and ÐPCB = Ð x / 2 From PC
sin PCB = PB \ ÐPCT = Ð x, therefore ÐPCA = 180° - x PA
= PC sin PCA \ a = y sin (180° - x) = y sin x
(2) We now solve (1) and (2)
for x, From (2), (3)/2x(1), x/2
» 54.74° x » 109.5° |
Consider the
right angled DPCB. Consider the
right angled DPCA. We apply
half-angle formula here. The fun is both
y and a are cancelled! |
||||
|
Technique We employ
the technique of setting two equations and solving simultaneous equations. If you cannot handle
half-angle formula, you can still find the optimal bond angle. Fun to try! |
|||||